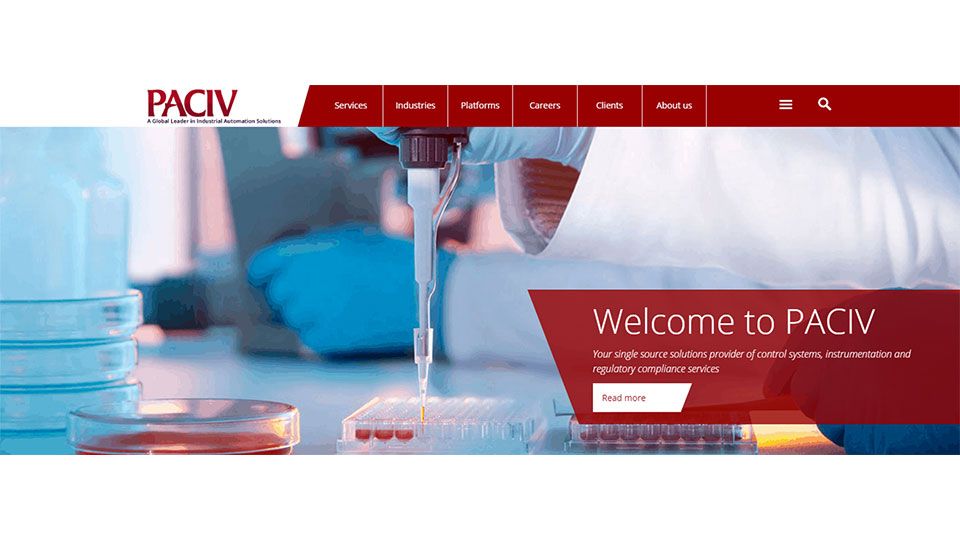
We Are Pleased to Announce the Launch of Our Brand-New Website
We are thrilled to announce the launch of our newly revamped website!
The design of the new website and the structure of information improves its overview and usability. The new design and colors now reflect the general PACIV image, makes it easier to navigate and is more user-friendly.
We wanted to give our clients the opportunity to know us better, who we are as a company and the leadership team behind our business. By browsing through our About Us and Our Leadership Team sections, you’ll get a pretty good idea of who we are and what we do. In the Accreditations & Memberships section, you’ll see what other are saying about us, our awards and partners.
You’ll find our new site is divided into six main sections: Services, Industries, Platforms, Careers, Clients and About Us....